25% of all additions to the Interchangeable List since 2013, have happened in the first 6 months of 2024.*
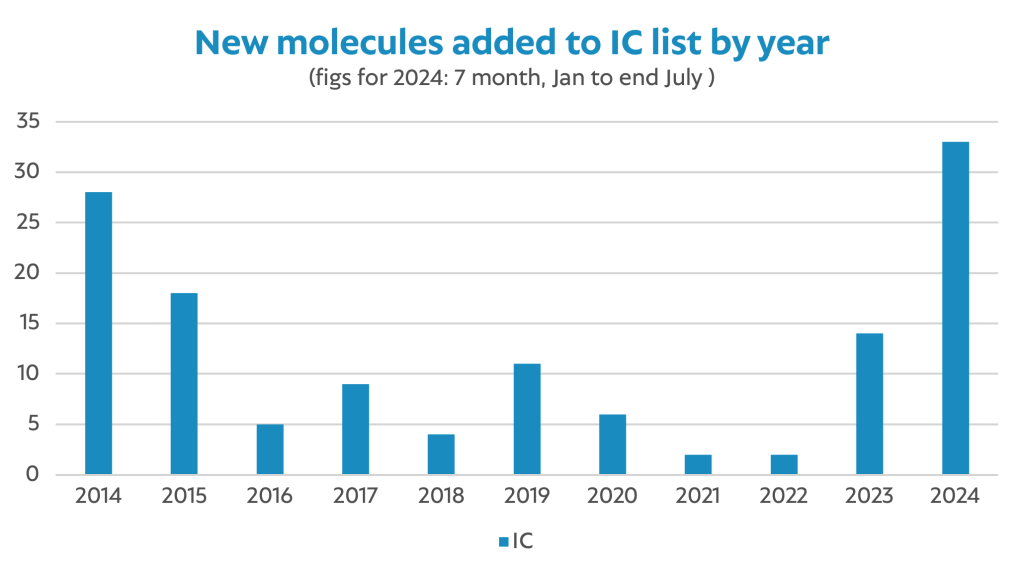
2024 has heralded a surge in additions to the Interchangeable List marking the busiest year of additions since the enactment of the Health (Pricing and Supply of Medical Goods) Act in 2013. Back then, under the watchful eye of the Troika, the Department of Health was under severe pressure to increase usage of generics from a paltry 8% by volume in order to maximise savings to the State’s medicines budget.
Between 2013 and 2014, 28 molecules were deemed to be interchangeable and over time, reference prices have been set and re-adjusted on multiple occasions, such that Ireland now has one of the lowest reimbursement prices for some of these medicines across the EU.
In 2024, the overspend in health and the requirement to make budget headroom for innovative medicines, as well as cover the organic growth in volume, has necessitated a renewed focus on the opportunity for savings that a greater use of generic medicines can deliver. In the year-to-date, some 33 medicines have been designated interchangeable. To put this in context, 25% of all additions to the Interchangeable List over the past decade have happened in the first six months of 2024. This trend further indicates the pressure on the budget and focus on cost savings.
Of particular note, is the number of High-Tech medicines that have now been added to the Interchangeable List, indicating that this is an area of focus for the HSE in terms of savings potential. The reference prices that have followed the interchangeable designation have been significantly reduced such that in the case of some strengths of Lenalidomide, the advised price will be roughly 11% of the pre-patent expiry price. The opportunity to drive significant savings in the High-Tech scheme seems to be in the sights of the HSE and given the growth in this scheme, it is little wonder.
